Pharma B2B doctor engagement videos 2026: The India Playbook for Personalized HCP Education and Growth
Estimated reading time: 17 minutes
Key Takeaways
- Hyper-personalized, mobile-first video is the preferred medium for HCP education in India in 2026.
- Ethical engagement hinges on UCPMP 2024 compliance, explicit consent, and robust MLR governance.
- A scalable doctor relationship personalization platform unifies data, automation, and low-latency rendering.
- Automation accelerates MoA explainers, device launches, and post-conference follow-ups while maintaining quality.
- Sustained growth requires retention programs, localized content, and transparent clinical-trial communications.
In the rapidly evolving landscape of the Indian pharmaceutical industry, pharma B2B doctor engagement videos 2026 have emerged as the definitive medium for bridging the gap between complex clinical data and actionable physician insights. As we navigate 2026, the traditional model of generic, high-volume broadcasting has been rendered obsolete by a sophisticated ecosystem that demands hyper-personalization, strict ethical adherence, and mobile-first delivery.
The inflection point for the Indian market is driven by three converging forces: the maturity of generative AI, the ubiquitous reach of WhatsApp Business at scale, and the rigorous enforcement of the Uniform Code for Pharmaceutical Marketing Practices (UCPMP) 2024. Today’s healthcare professionals (HCPs) in India are no longer passive recipients of information; they are digitally-savvy stakeholders who prioritize concise, evidence-led, and on-demand content that respects their time and clinical autonomy.
According to Express Pharma, AI-driven engagement and digital health personalization are the top priorities for a future-ready industry in 2026. This shift is further solidified by the UCPMP 2024 framework, which formalizes ethical promotion norms, making consent and transparency non-negotiable. This guide provides a comprehensive roadmap for implementing an enterprise-grade video strategy that balances high-impact engagement with absolute regulatory compliance.
1. The State of Healthcare Professional Video Marketing India in 2026
The current paradigm of healthcare professional video marketing India is defined by a move toward asynchronous, micro-learning modules that fit into the fragmented schedules of modern clinicians. Indian HCPs have demonstrated a higher degree of digital openness compared to many global peers, specifically favoring content that is delivered via familiar channels like WhatsApp and integrated CRM portals.
Research from Indegene’s Digitally-Savvy HCP report indicates that Indian physicians are increasingly seeking specialty-specific explainers that provide immediate clinical utility. In 2026, the most effective video assets are those ranging from 45 to 120 seconds, focusing on Mechanism of Action (MoA), dosing updates, and safety information (ISI) with high-quality closed captioning for silent viewing in clinical settings.
The delivery mechanisms have also shifted toward high-intent triggers. Rather than mass emails, successful campaigns now utilize CRM-triggered sends—such as those from Veeva or Salesforce—that respond to specific HCP actions like attending a conference session or requesting information on a new indication. IQVIA’s analysis confirms that asynchronous video is the preferred channel for deep-dive education, allowing HCPs to consume content at their convenience while maintaining a direct line to Medical Affairs.
Furthermore, localization has become a critical differentiator. As pharma brands expand their reach into Tier-2 and Tier-3 cities, the ability to provide video content in regional languages is no longer a luxury but a requirement for building trust. This localized approach ensures that educational nuances are not lost in translation, fostering a more inclusive and effective HCP engagement personalized content strategy.
2. Compliance and Consent Foundations for Healthcare Compliance Marketing India
Navigating the complexities of healthcare compliance marketing India requires a compliance-by-design approach, particularly under the stringent guardrails of UCPMP 2024. The core of this framework is the absolute prohibition of inducements, requiring that all engagement remains strictly educational and evidence-based.
To maintain compliance, every video asset must undergo a rigorous Medical, Legal, and Regulatory (MLR) review process. This includes the mandatory inclusion of fair-balance information and safety overlays within the video frames themselves, ensuring that risks are presented with the same prominence as benefits. Documentation is the bedrock of this process; every version of a video, along with its approval history and distribution log, must be stored in an audit-ready digital repository.
Consent management is the second pillar of a compliant strategy. In 2026, explicit, purpose-specific consent is required before any promotional or educational content is delivered via digital channels. This involves maintaining a centralized consent ledger that tracks when and how an HCP opted in, the specific topics they agreed to receive, and a seamless mechanism for them to opt out at any time.
Finally, the use of prescription influence marketing videos must be handled with extreme ethical care. The goal is to influence appropriate prescribing through improved clinician understanding of approved indications and dosing, rather than through material incentives. By aligning video content with the official UCPMP 2024 guidelines, pharma companies can build long-term credibility while mitigating the risk of regulatory penalties or reputational damage.
3. Your Doctor Relationship Personalization Platform Blueprint
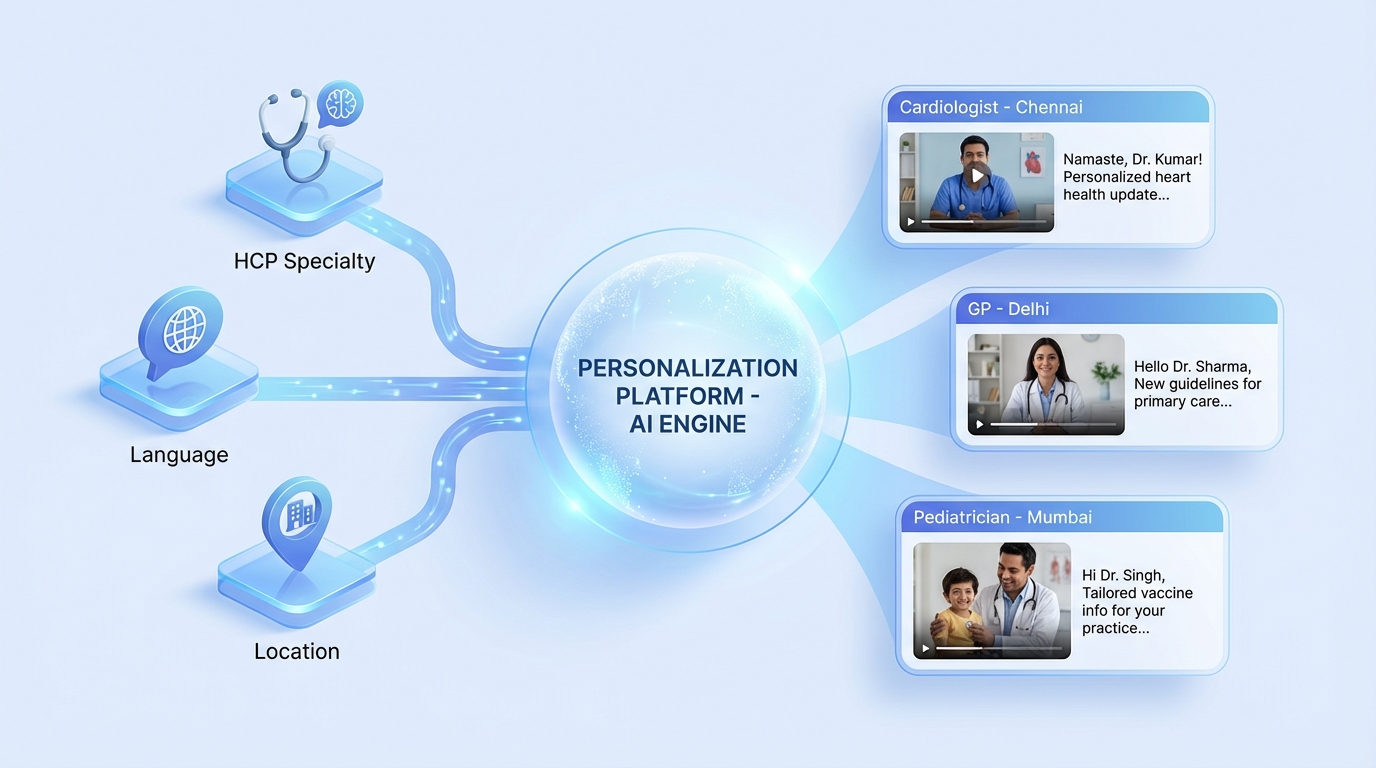
An enterprise-grade doctor relationship personalization platform is the engine that allows pharma brands to scale one-to-one engagement without a proportional increase in manual effort. This system integrates first-party data from CRMs, CDPs, and marketing automation tools to generate dynamic, compliant video sequences tailored to the individual HCP’s profile.
The core data inputs for such a platform include the HCP’s name, specialty, geographic location, preferred language, and historical engagement patterns. For instance, a cardiologist in Chennai might receive a video in Tamil discussing the latest clinical trial data for a heart failure medication, while a general practitioner in Delhi receives a Hindi-language explainer on dosing for the same product.
Platforms like TrueFan AI enable this level of granular personalization by utilizing modular video templates where specific fields—such as the greeting, the hospital name, or the call-to-action—are dynamically swapped during the rendering process. This ensures that every video feels bespoke to the recipient while maintaining the integrity of the core medical message approved by the MLR team.
The technical architecture must also support low-latency rendering and seamless API integrations. When a medical representative logs a meeting in Veeva, the platform should be able to trigger a personalized follow-up video within seconds. This real-time capability is essential for maintaining the momentum of the educational journey and ensuring that the HCP receives the right information at the exact moment of need.
4. Pharmaceutical Education Video Automation and Device Launches
The implementation of pharmaceutical education video automation allows medical affairs teams to transform static clinical data into dynamic learning assets. By authoring modular scripts that mark dynamic fields for indications, dose ranges, and safety updates, companies can produce hundreds of variations from a single base shoot.
This automation is particularly valuable for complex topics like Mechanism of Action (MoA) animations or dosing titration walkthroughs. When a new indication is approved, the platform can use virtual reshoots to update specific lines of dialogue or text overlays without requiring the original spokesperson to return to the studio. This significantly reduces production costs and time-to-market for critical updates.
In the context of medical device launch campaigns, personalized video serves as a bridge between technical specifications and procedural application. Pre-launch videos can feature KOL previews and virtual in-service training teasers tailored to the specific specialty of the investigator. During the launch phase, procedural setup guides and device differentiators can be delivered directly to the HCP’s mobile device, ensuring they have the necessary support for successful adoption.
Post-launch, the strategy shifts to training refreshers and troubleshooting guides. According to the TrueFan AI blog on medical device launches, using personalized video sequences to provide service reminders based on the installed base of a device can significantly improve user satisfaction and long-term loyalty. TrueFan AI's 175+ language support and Personalised Celebrity Videos further enhance this by allowing brands to leverage recognized voices for high-impact educational delivery.
5. Medical Conference Follow-up Automation and Rep Enablement
One of the most significant missed opportunities in pharma marketing is the post-conference lag. Medical conference follow-up automation solves this by triggering personalized video summaries within 24 to 48 hours of an event. By segmenting HCPs based on the sessions they attended or the booth interactions they had, brands can deliver next-best content that feels relevant and timely. See the ONDC Personalized Video Onboarding playbook for related orchestration patterns.
For example, an HCP who attended a symposium on oncology could receive a video featuring a KOL snippet from that specific session, along with a personalized CTA to download the full slide deck. This level of responsiveness demonstrates a commitment to the HCP’s professional development and keeps the brand top-of-mind in a crowded therapeutic area.
Furthermore, medical rep enablement videos are transforming hybrid selling models. Reps can now use a library of short, specialty-tailored clips to set the agenda before a call or handle specific objections afterward. These videos act as a digital leave-behind that is far more engaging than a standard PDF or email.
Solutions like TrueFan AI demonstrate ROI through increased meeting acceptance rates and higher engagement with approved content. When a rep sends a personalized video via WhatsApp that addresses a doctor by name and references their specific clinical interests, the likelihood of a meaningful follow-up interaction increases exponentially. This integration of human touch and digital scale is the hallmark of a successful 2026 commercial strategy.
6. Medical Professional Retention Strategies and Clinical Trials
Building long-term trust requires medical professional retention strategies that go beyond the product lifecycle. This involves creating a value exchange where the HCP receives ongoing educational support, early access to research briefings, and opportunities for peer-to-peer collaboration.
Doctor loyalty program automation can facilitate this by tracking engagement across multiple touchpoints and rewarding active participation with knowledge-based incentives. For instance, an HCP who consistently engages with educational videos might be invited to join an exclusive advisory board or a closed-door webinar with international experts. This approach fosters a sense of professional community and mutual respect, which is essential for sustained advocacy.
In the realm of clinical research, clinical trial recruitment videos India are becoming a vital tool for boosting enrollment ethically. These videos, which must be strictly non-promotional and approved by the relevant Ethics Committees (IEC/IRB), explain the study purpose, eligibility criteria, and safety oversight to potential investigators and referring physicians.
By personalizing these videos by site and region, sponsors can address the specific logistical concerns of local investigators, leading to faster site activation and improved screening-to-enrollment ratios. The focus here is on transparency and data minimization, ensuring that the recruitment process is as frictionless as it is compliant.
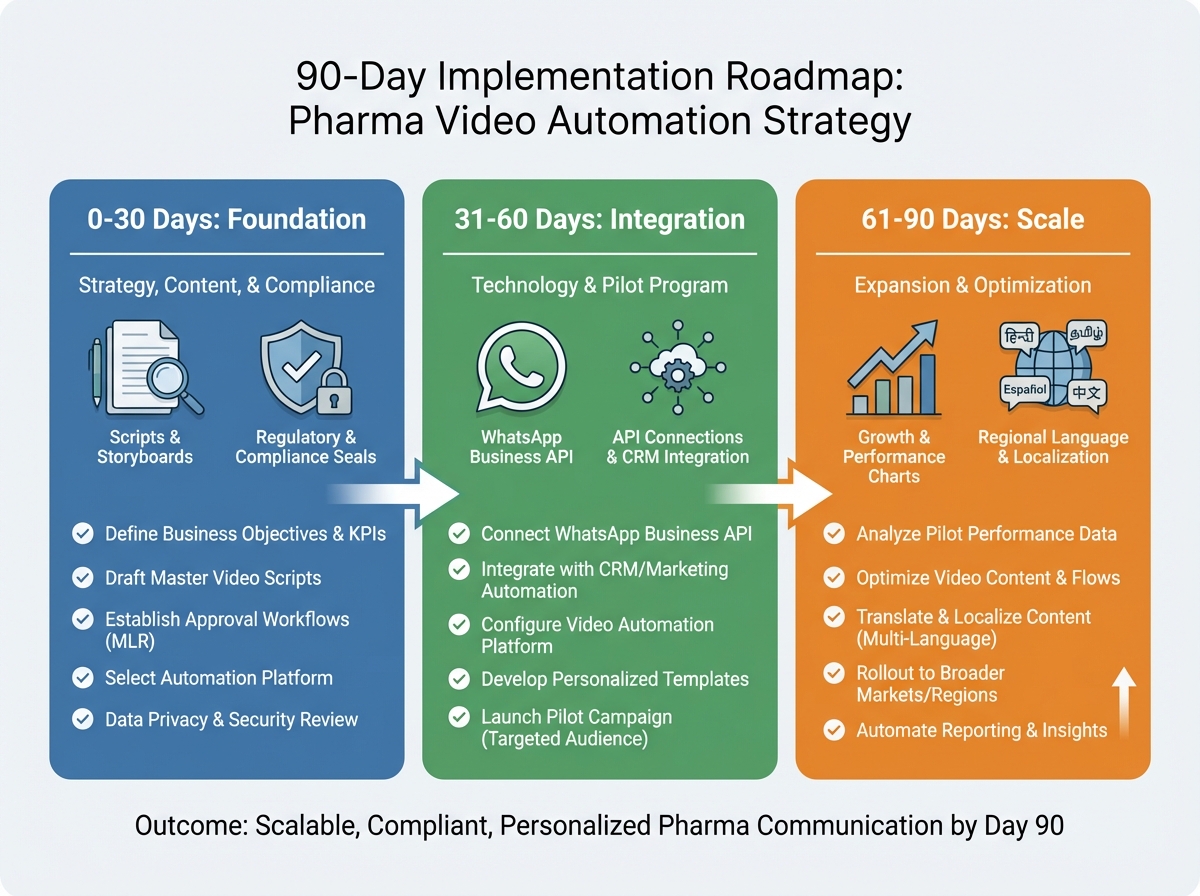
7. Implementation Roadmap for HCP Engagement Personalized Content
Successfully deploying a strategy for HCP engagement personalized content requires a structured 90-day implementation plan. This roadmap ensures that the technical, creative, and compliance workstreams are aligned for maximum impact. Explore adjacent planning guidance in Digital Transformation Budget Planning.
0–30 Days: Foundation and Design
- Identify the top 2–3 use cases, such as pharmaceutical education video automation and conference follow-up.
- Map the necessary data fields from the CRM, including consent status, specialty, and language preferences.
- Develop modular script templates and storyboards that include mandatory UCPMP-aligned disclaimers and fair-balance overlays.
31–60 Days: Integration and Pilot
- Establish API connections between the personalization platform and delivery channels like WhatsApp Business and Veeva.
- Launch a pilot program targeting a specific therapeutic area or geographic region (e.g., Tier-1 and Tier-2 cities in South India).
- Monitor initial KPIs such as watch time, completion rates, and consent opt-ins to refine the content and delivery logic.
61–90 Days: Scale and Optimization
- Expand the program to additional specialties and integrate clinical trial recruitment or doctor appreciation campaigns.
- Implement quarterly what’s new personalized update reels to maintain consistent engagement.
- Establish a governance cadence to review audit logs and ensure ongoing compliance with evolving regulatory standards.
Frequently Asked Questions
How do we ensure UCPMP compliance in personalized videos?
Compliance is maintained through pre-approved content libraries, mandatory fair-balance overlays, and automated audit logs that track every video generated and sent. All assets must pass through a standard MLR review process before being modularized for personalization.
What level of consent is required for WhatsApp delivery?
Under 2026 standards, HCPs must provide explicit, opt-in consent for receiving educational or promotional content via WhatsApp. This consent must be logged with a timestamp and include a clear description of the type of content to be shared.
Can these platforms integrate with our existing Veeva or Salesforce CRM?
Yes, enterprise solutions like TrueFan AI are designed to integrate via robust APIs and webhooks. This allows for trigger-based video rendering where an action in the CRM (like a meeting completion) automatically initiates the creation and delivery of a personalized video.
How do we measure the success of these videos without violating inducement rules?
Success is measured through content-value KPIs such as average watch time, completion rates, and clicks to medical inquiry forms. These metrics focus on the educational utility of the content rather than direct prescription attribution, ensuring ethical alignment.
How fast can we localize video content into regional Indian languages?
With automated personalization platforms, localization can be achieved almost instantly once the base templates and translated overlays are approved. TrueFan AI, for example, supports over 175 languages, allowing for rapid scaling across diverse linguistic regions in India.
By adopting a data-driven, video-first approach to HCP engagement, Indian pharma companies can navigate the complexities of 2026 with confidence. The combination of hyper-personalization, ethical compliance, and automated delivery creates a powerful framework for building lasting relationships with the medical community while driving sustainable business growth.
Sources:




